What makes us different
Clinigen are a full service, high quality global provider of Pharmacovigilance and Medical Information services operating from early development through to post marketing on a global scale. Putting a premium on deep experience, hands-on customer service and flexible methodologies our senior team with an average of more than 15 years' experience in the PVG & drug safety market – is engaged in clients projects every single day.
Clinigen understands that your organisation has unique needs, which is why a flexible engagement model for our clients is important. In some situations, work can be provided on a project basis and for other clients it may be as a fully-outsourced extension of their organisation, serving as their virtual safety department with global pharmacovigilance support for both clinical and post-marketed medical products.
Expertise
Clinigen have experience & expertise across many therapeutic areas & currently provides support to clients with products either authorised or under development within wide range of areas such as:
- Infectious diseases
- Pain Management
- Oncology Metabolic & Endocrine
- Hepatology Vascular Disorders
- Nephrology Gastrointestinal
- Neurology Pediatric and neonatal
- Genetic Gastroenterology
- Psychiatric Hematology
- Transplant and Immunology Ophthalmology & Cardiovascular
- Respiratory diseases
Leadership Team
Sarah joined Clinigen in 2016 and provides pharmacovigilance oversight across the Clinigen Group, both for the products for which Clinigen is the Marketing Authorisation Holder, as well as for PV Services provided to Clients for early access programmes. Prior to this she spent 17 years in multi-aspect Pharmacovigilance roles, starting her career at the MHRA, then working in a number of global positions for both small-medium and large Pharmaceutical companies.
Ricardo is a Drug Safety Physician with demonstrated expertise working in the pharmaceutical industry and successfully supporting several drugs through clinical development to post-marketing approval since starting with DSN in 2016. Ricardo has a strong background in clinical research and has experience working in several therapeutic areas such oncology, haematology, cardiology and neurology to name a few. Ricardo provides medical oversight, drug safety expertise, and leadership to ensure the delivery of quality & safety pharmacovigilance services for drugs, biologics, and medical devices. In addition, Ricardo is also responsible for leading the medical and literature reviews, ongoing signal detection, and risk minimization strategies, aggregate reports and regulatory authority communications.
Kristina has more than 13 years of experience with a diverse background. Her quality and performance driven background has led to her experience in building departments, creating/reorganizing and managing business units, mentoring staff and managing/attending executive meetings in order to outline strategic organizational path. Kristina joined the company in 2017 and has been responsible for overseeing the business’s strategic vision and leading business decisions to drive company growth. In addition to identifying potential risks and opportunities within the organization and its environment to protect business interests. Kristina is experienced in overseeing multiple departments to ensure consistency and cross-functional work.
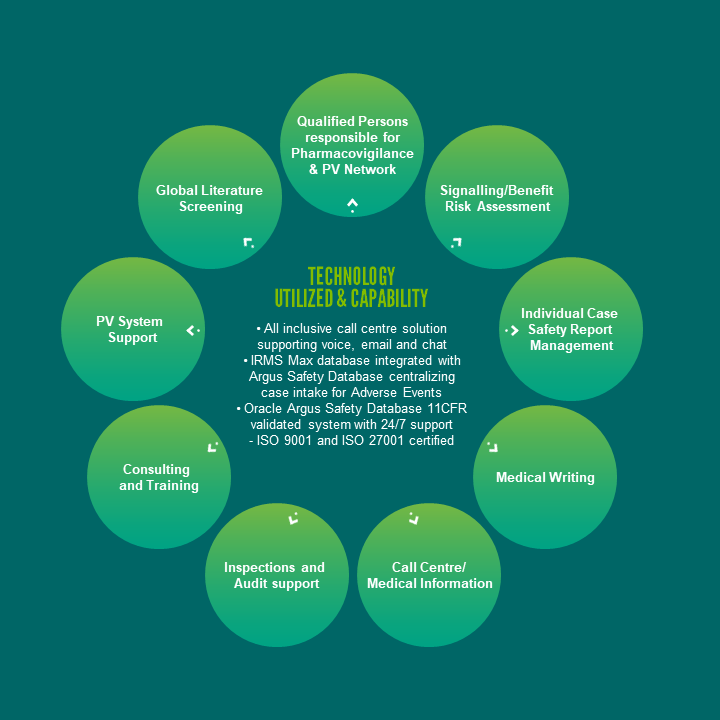
Pharmacovigilance Services
Clinigen’s QPPVs and deputies undergo a training program, share their experiences & best practices through an internal QPPV forum & are kept up-to-date with key changes to EU/UK PV legislation & guidelines. Outside the EEA Clinigen we can support an extensive network covering 100+ countries of skilled & up-to-date trained LCPPVs.
At Clinigen, our pharmacovigilance services ensure your safety signal management activities are comprehensive and our actions are always timely, sensitive, accurate and compliant.
Whether transitioning legacy safety data or supporting a client’s future needs for processing ICSRs our experienced pharmacovigilance physicians will review MedDRA coding and perform medical assessment of all cases. Clinigen’s Argus Oracle Health Sciences Safety system 21 CFR part 11 is a fully compliant safety solution, which is an essential part of Good Pharmacovigilance Practice.
This solution enables rapid data assessment for signal detection, aggregate report production, & statutory electronic reporting of cases to the regulatory authorities.
Our consultants & employees have years of experience of planning and writing aggregate reports including periodic safety update reports (PSURs), periodic adverse drug experience reports (PADERs) and development safety update reports (DSURs) in a cost-effective way. Whether it is a single simple DSUR or PADER or complex PSURs, we are able to meet your requirements.
Our team of healthcare professionals and life science degree graduates use industry leading call centre software together with IRMS Max, a validated, web-based, flexible, and adaptive enquiry and document management solution, to receive, document, track and respond to all medical information inquiries from Consumers, Healthcare Providers, and company Representatives.
In addition to medical information inquiries, all adverse events, special situation reports, and product quality complaints are also captured in IRMS Max. All safety information captured in IRMS Max is seamlessly transferred to pharmacovigilance, as our IRMS Max and Argus databases are integrated.
Clinigen has many years’ experience conducting pharmacovigilance audits worldwide and developing programmes with client companies to ensure that all elements of their pharmacovigilance system are appropriately covered.
We have experts within our team that have over 25 years’ experience in pharmacovigilance & medical information who continue to remain passionate, detail orientated & quality driven.
We work with their clients & can provide as much or as little strategic guidance as required by the client.
The high quality of provided pharmacovigilance consulting services is primarily ensured by these experts as well as PV & Medical Information (MI) professionals forming the leadership team.
With our Argus Oracle Health Sciences Safety system we support both PV and MI requirements and are flexible with our Clients while advising them on the set-up and management of the systems. We can offer solutions to minimize touch (E2B from IRMS to Argus), rapid data assessment from monthly signal detection to Aggregate reports. Utilising the Oracle Argus Safety Database in the Cloud. The system is hosted by a premier Life Science Hosting Administrator, who utilizes an ISO 9001-certified Quality Management System to deliver GxP compliant hosting across two dedicated Data Centres. With 24/7 support from our host, we can focus on case management from receipt to reporting without having to support a large IT infrastructure.
The scientific/medical literature is an key source of information for the safety profile of medicinal products, especially in the detection of new safety signals or emerging safety issues (ESI).
We operate a comprehensive Worldwide Pharmacovigilance Literature Screening Service as part of the company’s suite of drug safety services covering global and local literature screening.
We also perform global, and in some countries local, biomedical literature screening to identify Individual Case Safety Reports (ICSRs) and any information that could impact the benefit-risk profile of the product, which includes new safety signals for emerging safety issues.
Case Studies
- Clinical Safety Support for Bio-Pharmaceutical
Situation/Problem
Small, resource-limited Bio-Pharma with a novel and important drug for treatment and prevention of autoimmune conditions. Pre-clinical and Phase I studies in EU and US prior to Clinigen involvement. After positive feedback and suggestions for future indications to study from the regulatory authorities, the company moved into Phase II studies. Clinigen was requested to manage expanding company activities, and integrate all safety data into one global repository.
Solution
Clinigen now functions as a “virtual safety department” providing high-level strategic and tactical advice to ensure regulatory compliance, patient safety and forward-thinking strategies for ongoing clinical development and ultimate registration/drug approval.
Value to client
The company revised protocols in line with Clinigen’s recommendations, which will serve as a template for future studies. The Development Safety Update Report supplied by Clinigen provided a summary of important and potentially important risks and risk mitigation strategies. This provided the company with a summary of the product’s benefit-risk profile and a foundation for further ongoing benefit-risk assessments.