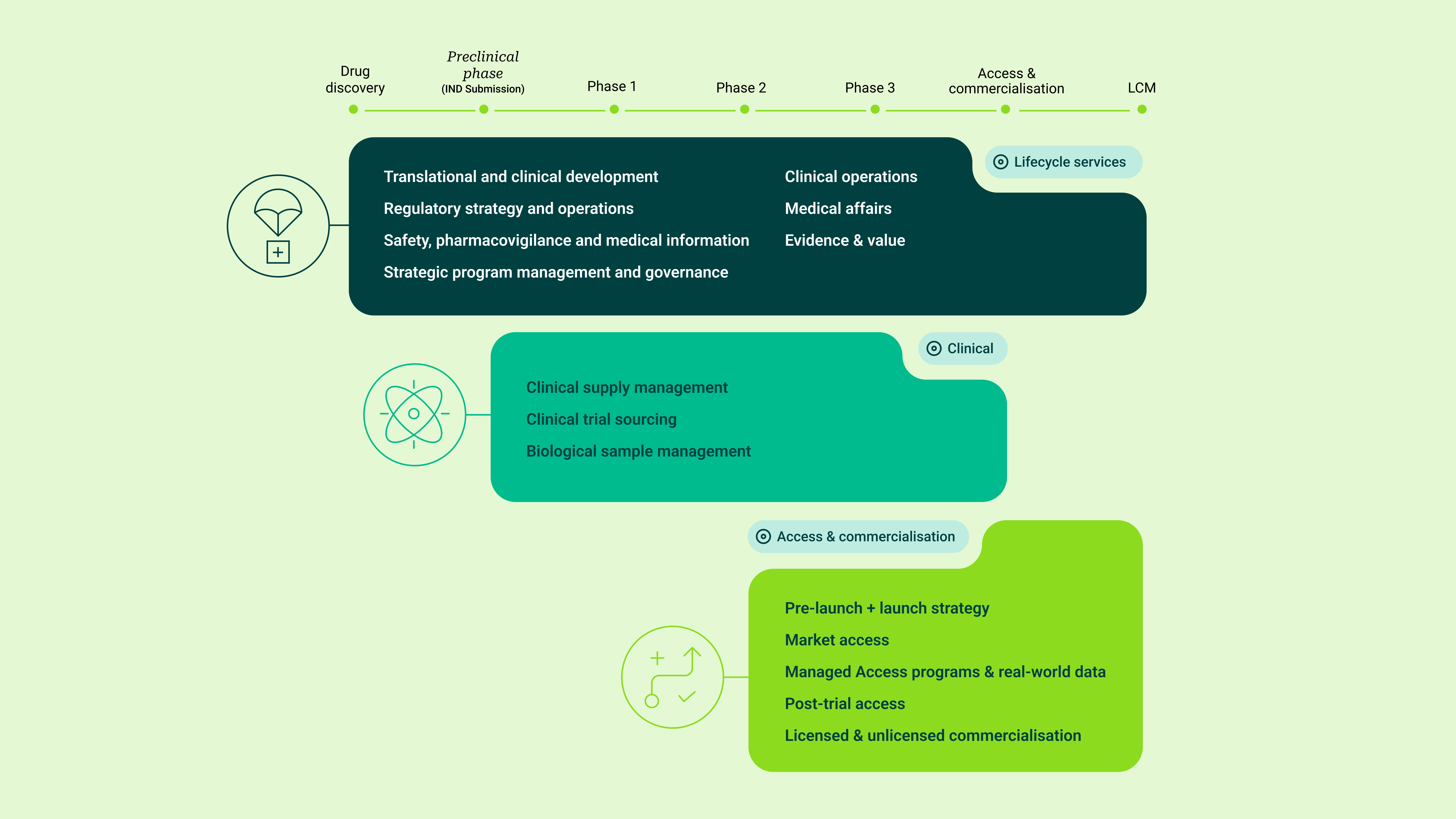
Partnering across the product lifecycle requires more than individual services. It demands coordination, continuity and deep operational understanding across development, access and commercialisation.
Clinigen partners with pharmaceutical and biotechnology companies to support access to critical medicines, bringing together clinical supply, regulatory, access and commercial capabilities within one integrated global platform.
How we work with partners
Our teams work as an extension of our partners’ organisations, combining global reach with strong local execution.
Integrated support from development through to post-launch access
Licensed and unlicensed pathways managed within a single framework
Regulatory, quality and safety oversight embedded throughout
Global logistics aligned with local market expertise

Leadership team
An experienced leadership team with deep expertise across global access, regulation and supply.
Meet our experts
Global presence
A global footprint spanning 14+ office locations, supporting partners across local healthcare systems.
Explore our locations
Purpose and values
We exist to help ensure patients around the world can access the medicines they need.
Discover our purpose